
What is Generative AI?
Generative AI is a type of artificial intelligence that creates new content, such as text, images, music, code, or even videos, by learning patterns from existing data. Instead of just analyzing or classifying data like traditional AI, generative AI produces something new and original. Generative AI refers to artificial intelligence models that can create new content, such as text, images, music, and even biological sequences, based on patterns learned from training data. These models, often powered by deep learning techniques like Generative Adversarial Networks (GANs) and Transformers, are capable of generating realistic and novel outputs.
How Does Generative AI Work?
Generative AI is powered by machine learning models, often using deep learning techniques like neural networks. Here’s a basic breakdown:
Training Phase:
- The AI is trained on massive datasets (e.g., text for chatbots, images for art, audio for music).
- It learns the patterns, structures, and relationships within the data.
Generation Phase:
- Once trained, the AI can generate new outputs based on what it has learned.
Role of Generative AI in Bioinformatics
Generative AI is transforming bioinformatics by enabling the generation, analysis, and optimization of biological data, including DNA sequences, protein structures, and drug molecules. Some key applications include:

Protein Structure Prediction & Design
- AI models like AlphaFold and RoseTTAFold use deep learning to predict protein structures with high accuracy.
- Generative AI can design novel proteins with specific properties, aiding in drug development and synthetic biology.
Drug Discovery & Molecular Design
- AI can generate new molecular structures with desired biological activities, speeding up drug discovery.
- Generative models help optimize drug candidates by predicting their interactions with target proteins.
DNA & RNA Sequence Generation
- AI can design synthetic DNA sequences for genetic engineering.
- It helps in creating gene-editing strategies by optimizing CRISPR-Cas9 guides.
Biomedical Image Synthesis & Analysis
- GANs generate realistic biomedical images for training AI models in disease detection.
- AI improves medical imaging techniques, such as CT scans and MRIs, by enhancing image quality.
Synthetic Data Generation for Research
- AI can generate realistic biological datasets, allowing researchers to train models without relying on sensitive patient data.
- This helps in privacy-preserving AI applications in healthcare.
Evolutionary Biology & Genomics
- AI models simulate genetic variations and evolutionary processes to understand how diseases evolve.
- It assists in phylogenetic analysis and predicting mutations.
How Generative AI Enhances Genomic Data Analysis?
Generative AI is revolutionizing genomic data analysis by improving sequence generation, variant prediction, data augmentation, and pattern recognition.

Here’s how it contributes:
1.DNA & RNA Sequence Generation
- Generative AI can design synthetic genomic sequences that mimic real DNA/RNA, useful for training models without using sensitive patient data.
- AI-assisted sequence synthesis aids in genetic engineering and synthetic biology, enabling the creation of novel genes with desired functions.
Example:
GANs (Generative Adversarial Networks) have been used to generate realistic human-like DNA sequences for genomic research.
2. Variant Prediction & Disease Association
AI models can predict genetic mutations and their potential impact on diseases, helping in early diagnosis and personalized medicine.
Transformer-based models like BERT for genomics analyze genome sequences and predict pathogenic mutations.
Example:
DeepVariant (by Google) uses AI to improve variant calling accuracy in DNA sequencing data.
3. Data Augmentation for Rare Genetic Variants
- Generative AI can simulate rare mutations that aren’t frequently observed in datasets, improving machine learning models used in genomics.
- This helps train AI models to recognize rare diseases and genetic disorders more effectively.
Example:
GANs for genomic augmentation help improve deep learning models that classify disease-associated mutations.
4. Enhancing Genome-Wide Association Studies (GWAS)
- AI can generate synthetic genomic datasets to increase sample size in GWAS, leading to more accurate identification of genes linked to diseases.
- This reduces bias and improves statistical power in studies with limited real-world samples.
Example:
Variational Autoencoders (VAEs) are used to learn latent genomic patterns and generate realistic genomic data for analysis.
5. AI-Driven Phylogenetics & Evolutionary Analysis
- Generative models can simulate evolutionary scenarios, predicting how genomes evolve over time.
- This helps in studying virus evolution (e.g., COVID-19 mutations) and tracking how diseases spread through populations.
Example:
AI-driven phylogenetic trees predict evolutionary relationships between organisms based on synthetic genomic data.

6. Epigenomics & Gene Regulation Analysis
- Generative AI can model epigenetic modifications (DNA methylation, histone modifications) to understand gene expression patterns.
- This helps identify biomarkers for cancer and other diseases.
Example:
Deep generative models analyze epigenomic data to predict gene regulation mechanisms.
7. Personalized Medicine & Gene Editing
- AI can optimize CRISPR-Cas9 guide RNAs, making gene-editing tools more precise.
- Generative models predict how genetic modifications affect personalized treatment strategies.
Example:
AI-generated CRISPR guide RNA sequences improve gene-editing accuracy in therapeutics.
Generative AI vs. Traditional Bioinformatics Methods
Generative AI is transforming bioinformatics by enhancing efficiency, accuracy, and discovery capabilities. Below is a comparison of Generative AI and Traditional Bioinformatics Methods across key aspects:
1. Data Analysis & Pattern Recognition
| Feature | Traditional Bioinformatics | Generative AI |
| Approach | Rule-based, statistical models | Deep learning, generative models |
| Efficiency | Requires extensive manual feature selection | Learns patterns automatically |
| Scalability | Limited to predefined algorithms | Scales well with big data |
| Example | BLAST for sequence alignment | Transformer-based models for sequence prediction |
Advantage of AI:
- Can analyze large-scale genomic data faster and extract hidden patterns that traditional methods may miss.
2. Genome Sequence Prediction & Generation
| Feature | Traditional Bioinformatics | Generative AI |
| Sequence Design | Manual curation, alignment-based approaches | AI-generated DNA/RNA/protein sequences |
| Mutation Prediction | Rule-based (e.g., GWAS studies) | AI models predict mutations & impacts |
| Data Augmentation | Limited ability to generate synthetic sequences | GANs & VAEs generate realistic sequences |
| Example | Phylogenetic methods for sequence evolution | Deep generative models predicting novel genes |
Advantage of AI:
- Designs novel sequences that do not exist in nature, aiding in synthetic biology and drug discovery.
3. Structural Biology & Protein Folding
| Feature | Traditional Bioinformatics | Generative AI |
| Protein Folding | Energy-based modeling (e.g., Rosetta) | AI-based (AlphaFold, RoseTTAFold) |
| Accuracy | Approximate, needs experimental validation | Highly accurate predictions |
| Computational Cost | High (simulation-heavy) | Lower (once trained) |
| Example | Homology modeling | AlphaFold predicting 3D protein structures |
Advantage of AI:
- Faster and more accurate protein structure predictions without relying on costly experiments.
4. Variant Calling & Disease Prediction
| Feature | Traditional Bioinformatics | Generative AI |
| Variant Calling | Rule-based, threshold-dependent methods | AI models detect complex variations |
| Disease Association | GWAS, regression models | Deep learning discovers novel links |
| Accuracy | Limited by dataset size | Learns from diverse datasets |
| Example | HMMs for mutation prediction | DeepVariant for variant calling |
Advantage of AI:
- Identifies subtle genetic variations linked to diseases more effectively than traditional statistical methods.
5. Drug Discovery & Molecular Design
| Feature | Traditional Bioinformatics | Generative AI |
| Molecular Docking | Simulations, rule-based modeling | AI-generated molecules with desired properties |
| Screening Speed | Slow, experimental screening required | Rapid virtual screening |
| Optimization | Iterative lab experiments | AI refines designs instantly |
| Example | AutoDock for ligand docking | AI models like ChemGAN for drug discovery |
Advantage of AI:
- Accelerates drug discovery by predicting novel compounds and optimizing molecules faster.
6. Evolutionary & Phylogenetic Analysis
| Feature Traditional Bioinformatics Generative AI Evolution Modeling Tree-based methods (e.g., Maximum Likelihood) AI simulates genome evolution Mutation Prediction Sequence comparison-based AI predicts future mutations Computational Cost High for large datasets More efficient after training Example MEGA software for phylogenetics AI-generated evolutionary models |
Advantage of AI:
- Predicts how species and viruses evolve, helping in pandemic preparedness and vaccine development
7. Medical & Clinical Genomics
| Feature | Traditional Bioinformatics | Generative AI |
| Personalized Medicine | Based on existing biomarkers | AI predicts treatment responses |
| Epigenomics | Analysis of methylation sites | AI uncovers regulatory patterns |
| Data Privacy | Requires real patient data | AI generates synthetic data for training |
| Example | Biomarker-based diagnostics | AI-driven disease risk prediction |
Advantage of AI:
- Enhances precision medicine by tailoring treatments based on individual genetic makeup.
Future Trends of Generative AI in Bioinformatics
Generative AI is rapidly evolving, and its impact on bioinformatics is expected to grow significantly. Here are the key future trends to watch:

1. AI-Driven Personalized Medicine
- Genomic-based personalized treatments will be enhanced by AI models predicting how an individual’s genetic makeup affects disease risks and drug responses.
- AI-guided gene therapy will optimize CRISPR and other genome-editing techniques.
Example: AI will generate patient-specific drug compounds and optimize dosages based on genetic profiles.
2. Advanced Protein & Drug Design
- Next-gen AI models will design novel proteins & biomolecules tailored for specific medical or industrial applications.
- AI-driven de novo drug design will reduce the need for experimental trial-and-error in pharmaceutical research.
Example: Beyond AlphaFold, new models will predict protein-ligand interactions for ultra-fast drug discovery.
3. AI-Generated Synthetic Genomes
- AI will generate synthetic DNA sequences with desired functions, enabling synthetic biology breakthroughs.
- Custom-designed microorganisms for biofuel production, pollution cleanup, and medicine manufacturing.
Example: AI-generated bacteria that self-adapt to hostile environments for bioremediation.
- Generative AI for Rare Disease Research
- AI will simulate rare genetic mutations that are underrepresented in real-world datasets, improving diagnostic models.
- Virtual patient simulations will accelerate orphan drug development for rare diseases.
Example: AI will generate synthetic patient data for diseases like Huntington’s and ALS to train ML models.
5. AI-Powered Evolutionary & Phylogenetic Analysis
- AI will predict how viruses and bacteria evolve helping prevent future pandemics.
- Evolutionary simulations will help design resistant crops for climate change adaptation.
Example: AI models will forecast viral mutations, aiding in vaccine design.
6. Generative AI in Epigenomics & Gene Regulation
- AI will uncover epigenetic modifications that influence diseases like cancer, Alzheimer’s, and metabolic disorders.
- Predicting how lifestyle and environment affect gene expression will become a reality.
Example: AI-driven personalized epigenetic therapy for reversing disease-linked DNA modifications.
7. AI-Enhanced Data Privacy & Security
- Synthetic genomic data generated by AI will allow research without exposing real patient data, ensuring privacy compliance.
- AI-driven homomorphic encryption will secure genomic databases while allowing advanced analysis.
Example: AI will generate fake but realistic genomic datasets for secure biomedical AI training.
8. AI-Augmented Bioinformatics Software & Automation
- Future bioinformatics pipelines will be AI-automated, reducing human intervention.
- Self-learning algorithms will refine genomic predictions dynamically as new data emerges.
Example: AI-powered “self-correcting” genome annotation tools will improve accuracy without manual intervention.
9. AI & Quantum Computing for Genomics
- Quantum AI will revolutionize genetic sequencing, reducing computation time from weeks to minutes.
- Ultra-complex protein folding and molecular interactions will be solved in record time.
Example: Quantum-AI hybrid models will crack genetic diseases by simulating entire cellular environments.
10. AI-Integrated Multi-Omics Analysis
- AI will unify genomics, proteomics, transcriptomics, metabolomics, and microbiome data for holistic insights.
- This will lead to next-level precision medicine, predicting disease trajectories years in advance.
Example: AI will map the entire cellular system to predict aging, cancer progression, and metabolic disorders.
Guide to Using Generative AI for Biological Data

Generative AI is transforming the field of biological data analysis by enhancing genomic research, protein modeling, drug discovery, and synthetic biology. This guide provides an overview of its applications, tools, and best practices for using Generative AI effectively.
Understanding Generative AI in Biological Data
Generative AI refers to machine learning models that can generate, modify, and optimize biological sequences and structures. It is particularly useful for:
- DNA & RNA sequence generation
- Protein structure prediction & design
- Drug discovery & molecular synthesis
- Synthetic data generation for biomedical research
Types of Generative AI Models in Bioinformatics
- GANs (Generative Adversarial Networks) – Used to generate synthetic biological datasets.
- VAEs (Variational Auto encoders) – Learn and generate new biological sequences.
- Transformers (e.g., BERT, GPT-like models) – Process and predict genetic variations.
Key Applications of Generative AI in Bioinformatics
A. Genomic Data Analysis & Synthesis
- Generating synthetic DNA/RNA sequences for research.
- Predicting mutations and evolutionary patterns.
- AI-assisted genome annotation to improve sequencing accuracy.
B. Protein Structure Prediction & Design
- Predicting 3D structures from amino acid sequences.
- Designing novel proteins for therapeutic and industrial use.
- Optimizing protein folding for drug-target interactions.
C. Drug Discovery & Molecular Design
- AI-generated small molecules with specific biological activity.
- Optimizing chemical structures for drug efficacy.
- Predicting interactions between drugs and biological targets.
D. Synthetic Data Generation for Biomedical Research
- Creating realistic, privacy-preserving biological datasets .
- Training AI models without real patient data .
- Enhancing disease detection models with synthetic images & genomics data.
Steps to Implement Generative AI in Biological Research
Step 1: Choose the Right AI Model
- For sequence generation → Use VAEs & Transformers
- For protein modeling → Use Deep Learning (AlphaFold, RosettaFold)
- For drug discovery → Use GANs & Reinforcement Learning
Step 2: Prepare and Preprocess Data
- Curate high-quality biological datasets (genomes, protein structures, etc.).
- Use standardized formats (FASTA for sequences, PDB for proteins).
- Ensure ethical compliance when using patient data (HIPAA, GDPR).
Step 3: Train & Optimize the Model
- Use cloud computing (Google Colab, AWS, GPU clusters) for efficiency.
- Fine-tune models with domain-specific dataset.
- Validate generated data against experimental results.
Step 4: Evaluate & Interpret AI-Generated Results
- Use biological benchmarks to compare AI outputs with real-world data.
- Apply statistical validation to check accuracy (e.g., sequence alignment scores, protein RMSD).
- Collaborate with domain experts for experimental validation.
Challenges & Ethical Considerations
- Data Quality Issues – AI models depend on high-quality biological datasets.
- Black-Box AI Problem – Lack of interpretability in deep learning models.
- Ethical Concerns – AI-generated genomic modifications must be carefully regulated.
Best Practices for Responsible AI Use in Bioinformatics
- Use open-source AI models for transparency.
- Follow bioethics guidelines (avoid unintended genetic modifications).
- Ensure data privacy by using synthetic datasets when possible
Challenges of Implementing Generative AI in Bioinformatics
Generative AI has immense potential in bioinformatics, but its implementation comes with significant challenges. These challenges range from data quality issues to ethical concerns and computational limitations.
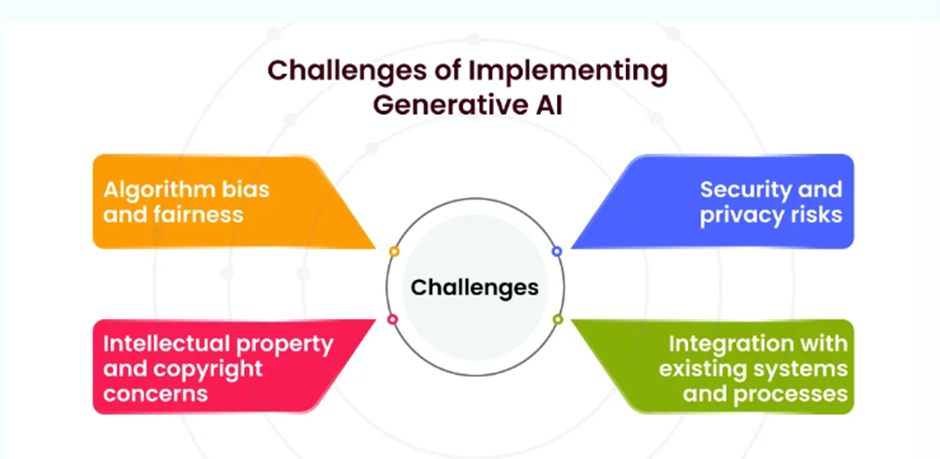
Data Quality & Availability
Challenge: AI models require large, high-quality biological datasets, but genomic and proteomic data often contain errors, biases, and missing values.
Key Issues:
- Incomplete or noisy data from sequencing technologies.
- Limited datasets for rare diseases, making AI training difficult.
- Standardization issues across different biological databases.
Solution:
- Use data augmentation techniques (GANs, synthetic data) to improve dataset diversity.
- Develop better data curation pipelines for pre-processing.
- Ensure cross-validation with experimental data.
Computational Complexity & Resource Requirements
Challenge: Generative AI models (e.g., AlphaFold, GANs) require high computational power (GPUs, TPUs, cloud resources), making them expensive to train and deploy.
Key Issues:
- Long training times for deep learning models.
- Limited access to high-performance computing (HPC) for smaller research labs.
- Energy consumption concerns with large AI models.
Solution:
- Use pre-trained models (e.g., AlphaFold Database) instead of training from scratch.
- Optimize AI algorithms for faster inference & lower memory usage .
- Leverage cloud computing platforms (Google Colab, AWS, IBM Watson).
Interpretability & Explainability
Challenge: AI-generated biological predictions often function as black boxes , making it difficult to interpret how the model arrived at a result.
Key Issues:
- AI-based genomic predictions need biological validation.
- Lack of explainability hinders clinical adoption in precision medicine.
- Regulatory challenges due to unclear AI decision-making.
Solution:
- Use explainable AI (XAI) methods (e.g., SHAP, LIME) to interpret AI outputs.
- Develop hybrid AI models combining deep learning with biological rules.
- Encourage AI-benchmarked experimental validation.
Future Trends in Generative AI for Biology
- AI-powered gene editing (enhanced CRISPR precision).
- AI-generated synthetic genomes for biotech applications.
- Quantum AI for faster genomic analysis.
CONCLUSION
Generative AI enhances traditional bioinformatics by providing faster, more accurate and scalable solutions for genomic analysis, drug discovery, and disease prediction. While traditional methods remain essential for validation and theoretical grounding, AI is pushing the boundaries of precision medicine and biotechnology. Generative AI has significantly transformed traditional bioinformatics by enabling researchers to analyze vast amounts of biological data more efficiently and accurately. It’s used for tasks like predicting protein structures, designing new drugs, analyzing gene sequences, and even discovering potential biomarkers for diseases. By leveraging techniques such as deep learning and natural language processing, generative AI can uncover patterns in biological data that might be missed by traditional methods alone. This synergy between AI and bioinformatics holds tremendous promise for advancing healthcare and biological research.
