In recent years, the intersection of generative artificial intelligence (AI) and bioinformatics has sparked tremendous innovation across various domains of biological research and healthcare. Generative AI, a subset of machine learning that focuses on creating new data from existing data, has revolutionized how molecular biology data is analysed interpreted, and applied. This blog explores the profound impact of generative AI in bioinformatics, highlighting key generative AI applications, challenges, and future directions.

Understanding Generative AI
Generative AI refers to a class of algorithms that learn to mimic or generate new data that resembles the input data they were trained on. Unlike traditional AI models that are designed for classification or prediction tasks, generative models can produce new examples that are indistinguishable from real data. This capability has proven invaluable in bioinformatics, where large-scale datasets are common and complex patterns need to be extracted and understood.
KEY GENERATIVE AI APPLICATIONS IN BIOINFORMATICS
1. Drug Discovery and Development
One of the most promising generative AI applications in bioinformatics is in drug discovery and development. Pharmaceutical companies are leveraging generative models to design novel compounds with specific therapeutic properties. These models analyze vast databases of chemical structures and predict which molecules are most likely to have desired molecular biology effects, accelerating the drug discovery process significantly.
Examples:
- GANs & VAEs for Molecular Generation: These models design molecules with specific therapeutic properties.
- AI-driven Drug Screening: AI predicts how compounds interact with target proteins.
- De Novo Drug Design: AI generates entirely new drug molecules from scratch.
Impact: Faster identification of potential drugs leading to drug discovery, reduced failure rates in clinical trials.
2. Genomics and Sequence Analysis
In genomics, generative AI plays a crucial role in deciphering DNA sequences and understanding their functional implications. Sequencing technologies generate enormous amounts of genomic data, which can be overwhelming to analyze manually. Generative models, such as recurrent neural networks (RNNs) and transformer-based architectures like GPT (Generative Pre-trained Transformer), are employed to predict and generate sequences that conform to molecular biology constraints.
These models aid in tasks such as genome assembly, variant calling, and predicting protein structures from amino acid sequences. By generating synthetic sequences, researchers can simulate mutations, understand evolutionary patterns, and design experiments to validate hypotheses about genetic function and disease susceptibility.
Examples:
- Synthetic DNA/RNA Sequence Generation: AI generates novel gene sequences for research and therapy.
- Variant Effect Prediction: AI predicts the impact of mutations on genes and proteins.
- CRISPR Guide RNA Optimization: AI helps design guide RNAs for precise genome editing.
Impact: Improves genetic research, facilitates personalized medicine, and enhances gene therapy approaches.
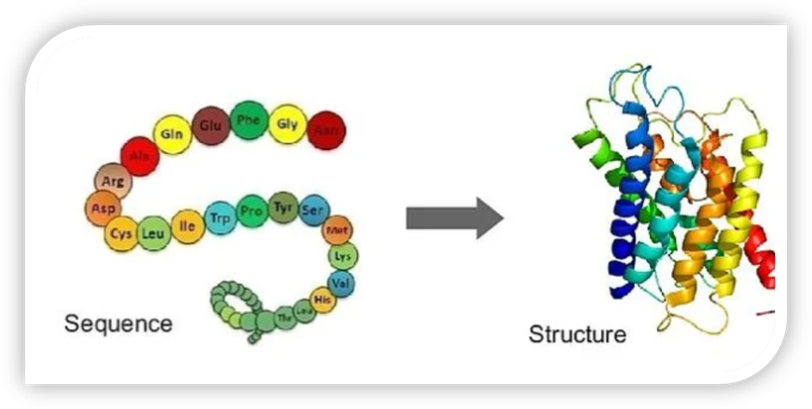
3. Protein Structure Prediction
Predicting the 3D structure of proteins is a fundamental challenge in bioinformatics, critical for understanding their functions and interactions with other molecules. Generative AI techniques, particularly deep learning models like AlphaFold, have made significant strides in accurately predicting protein structures from amino acid sequences.
AlphaFold, developed by DeepMind, uses a deep learning architecture trained on vast databases of known protein structures to predict the folding patterns of new proteins. This breakthrough has the potential to revolutionize drug discovery, as it enables researchers to identify binding sites for small molecules and design therapeutics that interact with specific protein targets more effectively.
Examples:
- AlphaFold by DeepMind: Predicts protein folding structures using deep learning.
- RosettaFold: Uses AI to model protein-protein interactions.
Impact: Helps in drug-target identification, enzyme engineering, and understanding diseases at the molecular level.
4. Medical Image Analysis
Medical imaging generates complex datasets that require precise analysis for diagnosis and treatment planning. Generative AI models, such as convolutional neural networks (CNNs) and variational autoencoders (VAEs), are employed to enhance the resolution of images, segment organs or tumours, and generate synthetic images for training purposes.
These models can learn from labelled datasets to identify patterns indicative of disease or abnormality in medical images, assisting radiologists and clinicians in making accurate diagnoses. Moreover, generative models can simulate variations in imaging data, providing robust datasets for training AI algorithms without relying solely on scarce or privacy-sensitive medical data.
Examples:
- GANs for Image Synthesis: Generates synthetic medical images to train AI models.
- AI-powered MRI & CT Enhancements: Improves image resolution and noise reduction.
- Tumor and Disease Detection: AI analyzes images to identify cancer, Alzheimer’s, etc.
Impact: Improves diagnostic accuracy and enables AI training without needing extensive real-world datasets.
5. Personalized Medicine
Personalized medicine aims to tailor medical treatment to individual characteristics, such as genetic makeup, lifestyle, and environmental factors. Generative AI facilitates the integration of diverse data sources—genomic, clinical, and environmental—to predict patient outcomes and optimize treatment strategies.
By analyzing large-scale datasets and generating personalized predictions, AI models can assist clinicians in selecting the most effective therapies based on an individual’s genetic profile and disease characteristics. This approach not only improves treatment efficacy but also reduces adverse effects and healthcare costs associated with trial-and-error approaches.
Examples:
- AI-driven Biomarker Discovery: Identifies genetic markers for diseases.
- Personalized Drug Response Prediction: AI predicts how individuals will respond to specific drugs.
- Synthetic Patient Data Generation: AI generates anonymized patient data for research and clinical trials.
Impact: Reduces adverse drug reactions and enhances treatment efficacy.
6. Synthetic Data Generation for Research
Generative AI creates synthetic molecular biology datasets that help in model training, hypothesis testing, and data augmentation.
Examples:
- AI-simulated Genetic Data: Helps train models without using real patient data.
- Synthetic Microbial Communities: AI generates microbial interaction models.
Impact: Addresses data scarcity and privacy concerns while enabling more robust AI model development.
7. Evolutionary Biology and Systems Biology Modeling
Generative AI models molecular biology evolution, genetic variations, and interactions within biological systems.
Examples:
- Evolutionary Pathway Prediction: AI simulates how genetic mutations evolve over time.
- Cell Behavior Modeling: AI predicts cell interactions and responses to stimuli.
Impact: Advances evolutionary research and aids in synthetic biology applications.
CHALLENGES AND CONSIDERATIONS IN THE GENERATIVE AI APPLICATION IN BIOINFORMATICS
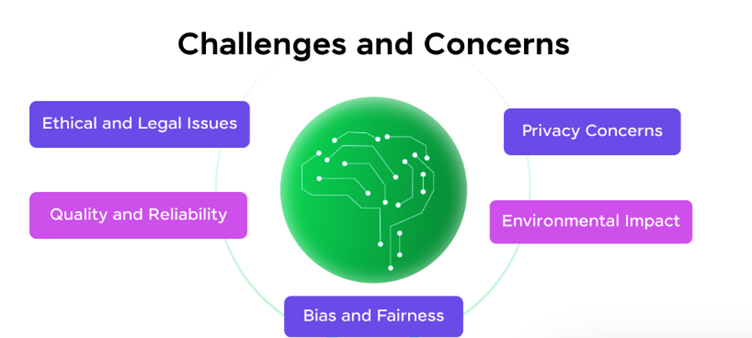
While generative AI is transforming bioinformatics, several challenges and considerations must be addressed to ensure effective and ethical generative AI applications. These challenges range from data-related issues to regulatory concerns and computational limitations. Below are some key obstacles and considerations:
1. DATA CHALLANGES
a. Data Quality and Availability
- Generative AI models require large, high-quality datasets for training.
- Many molecular biology datasets are noisy, incomplete, or biased.
- Some biological data is difficult to obtain due to privacy and ethical restrictions.
Solution: Improve data collection methods, use data augmentation techniques, and apply rigorous pre-processing.
b. Data Privacy and Security
- Genetic and medical data are highly sensitive.
- AI-generated synthetic data could still carry identifiable patterns.
Solution: Implement privacy-preserving techniques (e.g., differential privacy, federated learning) to ensure compliance with regulations like GDPR and HIPAA.
2. MODEL ACCURACY AND INTERPRETABILITY
a. Black-Box Nature of AI Models
- Many AI-generated molecular biology predictions lack explainability.
- Scientists struggle to understand how AI reaches its conclusions.
Solution: Develop interpretable AI models and integrate explainable AI (XAI) techniques.
b. Generalization Issues
- AI models trained on one dataset may not generalize well to others.
- Variability in molecular biology data (e.g., between populations) can reduce accuracy.
Solution: Use diverse datasets and rigorous cross-validation methods.
3. COMPUTATIONAL AND RESOURCE CONSTRAINTS
a. High Computational Costs
- Training deep learning models requires massive computational power.
- Not all research labs have access to high-performance computing (HPC) resources.
Solution: Use cloud-based AI solutions and optimize model architectures for efficiency.
b. Scalability Issues
- Scaling AI models for large-scale genomic and proteomic datasets remains a challenge.
Solution: Implement distributed computing and efficient data processing pipelines.
4. ETHICAL AND REGULATORY CONCERNS
a. Ethical Use of AI in Healthcare
- AI-generated medical decisions must be ethically sound.
- Biases in training data could lead to disparities in healthcare outcomes.
Solution: Establish ethical guidelines and continuously audit AI models for bias.
b. Regulatory and Compliance Issues
- AI in bioinformatics must comply with regulations like FDA (for drug discovery), GDPR (for data privacy), and HIPAA (for medical records).
Solution: Collaborate with regulatory bodies to develop AI governance frameworks.
5. POTENTIAL FOR MISUSE
a. Bioterrorism and Synthetic Biology Risks
- AI can be misused to generate harmful biological sequences (e.g., viruses, pathogens).
Solution: Implement strict access control, ethical oversight, and AI safety measures.
b. Intellectual Property Challenges
- AI-generated discoveries (e.g., drug discovery, genes) raise questions about patent rights and ownership.
Solution: Clarify legal frameworks around AI-generated biological innovations.
FUTURE DIRECTIONS
Looking ahead, the integration of generative AI with other emerging technologies, such as quantum computing and multi-omics data integration, holds promise for advancing precision medicine and understanding complex biological systems. Collaborations between researchers, clinicians, and AI developers will be crucial in harnessing the full potential of generative AI to address pressing challenges in bioinformatics.
CONCLUSION
Generative AI is revolutionizing bioinformatics by enabling breakthroughs in drug discovery, genomics, protein structure prediction, medical imaging, and personalized medicine. Its ability to generate new molecular biology data, predict complex patterns, and accelerate research is transforming how scientists understand and manipulate biological systems.
Despite its immense potential, challenges such as data privacy, model interpretability, computational costs, and ethical considerations must be addressed to ensure responsible and effective generative AI applications. With advancements in AI models, improved regulatory frameworks, and increased collaboration between AI researchers and biologists, generative AI is poised to become an indispensable tool in modern bioinformatics.
As we move forward, leveraging generative AI in bioinformatics will pave the way for faster drug discovery & development, more accurate disease diagnostics, and truly personalized medicine, ultimately improving healthcare outcomes and advancing scientific discovery.
